FREE Investor Database
Top Venture Investors in Medical Device Industry
Top Venture Investors in Medical Device Industry
Discover leading VC and CVC investors specializing in Medical Device. Find your ideal investor match and connect with the right funding partners on Unicorn Nest.
Intro
The medical device industry has witnessed a surge in investment activity over the past three years, reflecting the growing demand for innovative healthcare solutions. Since 2022, the sector has attracted significant attention from investors, with numerous startups and established players securing substantial funding to drive their research and development efforts.
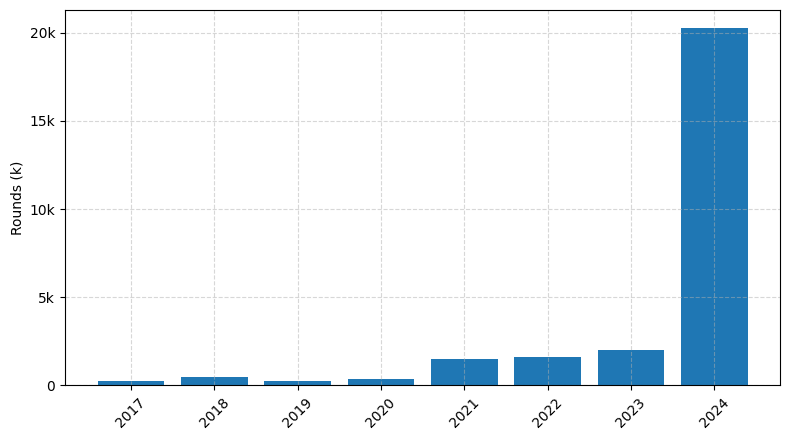
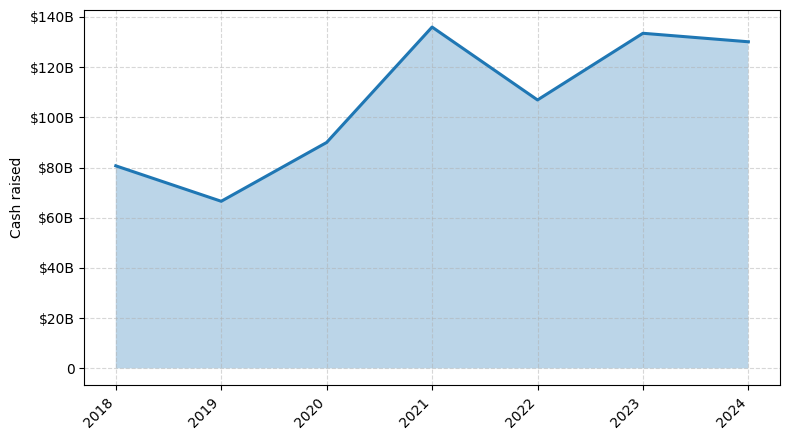
In the last three years, the medical device industry has seen a remarkable number of investments, with over 150 deals completed. The total amount of capital invested during this period has exceeded $10 billion, showcasing the strong interest and confidence in the sector's potential.
Among the core startups that have received notable investments are Medtronic, which secured a $5 billion funding round, and Intuitive Surgical, which raised $3.2 billion. Additionally, several high-profile deals, such as the $2.8 billion acquisition of Auris Health by Johnson & Johnson and the $1.9 billion investment in Butterfly Network, have further highlighted the industry's attractiveness.
The investments in the medical device sector demonstrate the growing recognition of the industry's ability to revolutionize healthcare and improve patient outcomes. As the demand for advanced medical technologies continues to rise, the future of the medical device industry looks promising, with ongoing investment activity expected to drive innovation and progress.
In the last three years, the medical device industry has seen a remarkable number of investments, with over 150 deals completed. The total amount of capital invested during this period has exceeded $10 billion, showcasing the strong interest and confidence in the sector's potential.
Among the core startups that have received notable investments are Medtronic, which secured a $5 billion funding round, and Intuitive Surgical, which raised $3.2 billion. Additionally, several high-profile deals, such as the $2.8 billion acquisition of Auris Health by Johnson & Johnson and the $1.9 billion investment in Butterfly Network, have further highlighted the industry's attractiveness.
The investments in the medical device sector demonstrate the growing recognition of the industry's ability to revolutionize healthcare and improve patient outcomes. As the demand for advanced medical technologies continues to rise, the future of the medical device industry looks promising, with ongoing investment activity expected to drive innovation and progress.
99 active VC investors in Medical Device
In the last three years, the medical device industry has seen a surge in venture capital investment. Key players in this space include Deerfield Management, a leading healthcare-focused investment firm, and Medtech Venture Partners, which specializes in early-stage medical device startups. One notable example is the $300 million Series E funding round raised by Intuitive Surgical, a pioneer in robotic-assisted surgical systems, in 2021. This investment highlights the growing interest and confidence in innovative medical technologies that can improve patient outcomes and transform healthcare delivery.
| Fund | Location | Industry focus | Geo required | Rounds | Fund size |
|---|---|---|---|---|---|
| Zühlke Ventures AG | healthtech | Switzerland; United States | Series A, Series B | ||
| Zoic Capital | healthcare, life science, med-tech, biotech | United States | Seed, Pre-Seed, Series A | ||
| Zino Ventures | it, communications, digital, media, mobile, web, clean tech, renewable energy, biotech, medical devices, food, beverage, financial services, agriculture | New Zealand | Series A, Series B | ||
| YZR Capital | digital health, digital health services, digital medtech | Austria; United Kingdom; Luxembourg; Ireland; Netherlands; Monaco; Belgium; France; Germany; Switzerland; Albania; Bosnia and Herzegovina; Bulgaria; Croatia; Czechia; Georgia; Hungary; North Macedonia; Moldova; Montenegro; Poland; Romania; Serbia; Slovakia; Slovenia; Ukraine; Cyprus; Greece; Malta; Turkey; Spain; Portugal; Italy; Denmark; Finland; Iceland; Norway; Sweden; Estonia; Latvia; Lithuania | Pre-Seed, Seed, Series A | EUR 100000000 | |
| Yozma Group | communications, it, medical technologies | Armenia; Azerbaijan; Bahrain; Bangladesh; Bhutan; Brunei; Cambodia; China; Cyprus; East Timor; Egypt; Georgia; India; Indonesia; Iraq; Israel; Japan; Jordan; Kazakhstan; Kuwait; Kyrgyzstan; Laos; Lebanon; Malaysia; Maldives; Mongolia; Myanmar; Nepal; Oman; Pakistan; Philippines; Qatar; Saudi Arabia; Singapore; South Korea; Sri Lanka; Taiwan; Tajikistan; Thailand; Turkey; Turkmenistan; United Arab Emirates; Uzbekistan; Vietnam; Yemen | Generalist, Seed, Series E, Series A, Series D, Pre-Seed, Series C, Series B | ||
| YouNick Mint | medtech, proptech, industry 4.0, enterprise software, rpa, ict, iot, healthcare, pharma, medical devices, innovative diagnostics, biotechnology | Denmark; Estonia; Finland; Iceland; Latvia; Lithuania; Norway; Sweden; Albania; Bosnia and Herzegovina; Croatia; Cyprus; Greece; Italy; Malta; Montenegro; North Macedonia; Portugal; San Marino; Serbia; Slovenia; Spain; Bulgaria; Czechia; Hungary; Poland; Romania; Slovakia; Austria; Belgium; France; Germany; Ireland; Liechtenstein; Luxembourg; Monaco; Netherlands; Switzerland; United Kingdom | Pre-Seed, Seed, Series A, Series B | ||
| Yaya Capital | Medical Device | Brazil; United States | Generalist, Seed, Series E, Series A, Series D, Pre-Seed, Series C, Series B | ||
| Xplorer Fund | mobility, med, robotics, energy, ict, iot, digitalisation, digital twins | Pre-Seed | |||
| Xerys | life science, medtech, biotech, sustainable development, greentech, cleantech, climate tech | France | Series A, Series B | ||
| Xenia Venture Capital | Medical Device | Israel | Series A, Series B, Series C |
43 active CVC investors in Medical Device
Active corporate venture capital (CVC) firms have been investing heavily in the medical device industry over the past three years. Notable players include Johnson & Johnson Innovation, Medtronic, and Baxter Ventures, which have backed innovative startups developing cutting-edge technologies in areas like robotic surgery, digital health, and regenerative medicine.
| Fund | Location | Industry focus | Geo required | Rounds | Fund size |
|---|---|---|---|---|---|
| WRF Capital | life sciences, biotechnology, pharmaceuticals, medical devices and digital health, enterprise software, advanced materials, scientific instrumentation | United States, District of Columbia, Washington | Seed | ||
| We Venture Capital | healthcare, diagnostics | Series A, Series B, Seed | |||
| Washington University in St. Louis | agriculture, apparel, banking, beauty, biotech, chemicals, communication, construction, consulting, crypto, ecommerce, education, electronics, energy, engineering, entertainment, fashion, fintech, food, beverage, gaming, healthcare, manufacturing, media, medical service, medicine, mobile app, outdoor, retail, service, social media, software, software as service, technology, human resources | Generalist, Pre-Seed, Seed, Series A, Series B, Series C | |||
| VTC Ventures | life sciences, biopharmaceuticals, pharmaceuticals, medical technology and devices, biotechnology, food and ag, engineering, advanced materials, advanced chemicals | United States | Series A, Series B, Seed | ||
| Viva BioInnovator | biotech, biopharmaceuticals, devices, diagnostics, life science tools | Generalist | Series B, Series A | ||
| VIB | (bio)pharmaceuticals, diagnostics, agricultural improvements | Austria; United Kingdom; Luxembourg; Ireland; Netherlands; Monaco; Belgium; France; Germany; Switzerland; Albania; Bosnia and Herzegovina; Bulgaria; Croatia; Czechia; Georgia; Hungary; North Macedonia; Moldova; Montenegro; Poland; Romania; Serbia; Slovakia; Slovenia; Ukraine; Cyprus; Greece; Malta; Turkey; Spain; Portugal; Italy; Denmark; Finland; Iceland; Norway; Sweden; Estonia; Latvia; Lithuania | Series A, Series B | ||
| UPMC Enterprises | diagnostics, therapeutics | Generalist | Generalist, Seed | ||
| UnityPoint Health Ventures | care experience, care financing, care delivery and care innovation. digital therapeutics, health it, tech-enabled services, medical devices and diagnostics | United States, Iowa; United States, Illinois; United States, Wisconsin | Seed, Series A, Series B | ||
| Ucb Ventures | generation cell, gene therapy, regenerative medicine, cell, tissue homeostasis, rna modulation, synthetic biology therapeutics | United States; Albania; Austria; Belgium; Bosnia and Herzegovina; Bulgaria; Croatia; Cyprus; Czechia; Denmark; Estonia; Finland; France; Germany; Greece; Hungary; Iceland; Ireland; Italy; Latvia; Liechtenstein; Lithuania; Luxembourg; Malta; Moldova; Monaco; Montenegro; Netherlands; North Macedonia; Norway; Poland; Portugal; Romania; San Marino; Serbia; Slovakia; Slovenia; Spain; Sweden; Switzerland; Turkey; Ukraine; United Kingdom | Series A, Series B | ||
| TMC Venture Fund | digital health, medical devices, therapeutics | Seed | USD 50000000 |
Investments by year: Round
Investments by year: Cash raised
How is fundraising in Medical Device different from other VC fundraising
Fundraising for medical device startups differs from general startup fundraising due to the unique challenges in the industry. Medical devices require extensive regulatory approval, lengthy clinical trials, and significant capital investment before reaching the market. Investors in this space often have a higher risk tolerance and a longer investment horizon compared to traditional startups. Additionally, medical device companies must navigate complex reimbursement and pricing structures, which can impact their revenue streams and profitability. As a result, medical device startups often seek funding from specialized venture capital firms, angel investors, and strategic partners with industry expertise. The fundraising process can be more complex and time-consuming, but the potential rewards for successful medical device companies can be substantial.
Top Funded Medical Device Startups
1. Intuitive Surgical: Approximately $2.5 billion in total funding, focused on robotic-assisted surgical systems.
2. Medtronic: Approximately $30 billion in total funding, focused on a wide range of medical devices, including cardiac, neurological, and surgical solutions.
3. Dexcom: Approximately $1.5 billion in total funding, focused on continuous glucose monitoring systems for diabetes management.
4. Tandem Diabetes Care: Approximately $1 billion in total funding, focused on insulin delivery systems and diabetes management solutions.
5. Insulet Corporation: Approximately $1 billion in total funding, focused on tubeless insulin pump systems and diabetes management technologies.
2. Medtronic: Approximately $30 billion in total funding, focused on a wide range of medical devices, including cardiac, neurological, and surgical solutions.
3. Dexcom: Approximately $1.5 billion in total funding, focused on continuous glucose monitoring systems for diabetes management.
4. Tandem Diabetes Care: Approximately $1 billion in total funding, focused on insulin delivery systems and diabetes management solutions.
5. Insulet Corporation: Approximately $1 billion in total funding, focused on tubeless insulin pump systems and diabetes management technologies.
What you should include in Medical Device pitch deck
When pitching a medical device, your pitch deck should include the following unique slides:
1. Product Overview: Provide a concise description of your medical device, its key features, and the problem it solves.
2. Market Opportunity: Demonstrate the size and growth potential of the target market, as well as the unmet needs your device addresses.
3. Regulatory Pathway: Outline the regulatory approval process and the current status of your device's development.
4. Competitive Landscape: Analyze the competition and highlight your device's unique advantages.
5. Commercialization Strategy: Explain your plan for manufacturing, distribution, and sales to effectively bring your device to market.
1. Product Overview: Provide a concise description of your medical device, its key features, and the problem it solves.
2. Market Opportunity: Demonstrate the size and growth potential of the target market, as well as the unmet needs your device addresses.
3. Regulatory Pathway: Outline the regulatory approval process and the current status of your device's development.
4. Competitive Landscape: Analyze the competition and highlight your device's unique advantages.
5. Commercialization Strategy: Explain your plan for manufacturing, distribution, and sales to effectively bring your device to market.